ModelView: Compact display of parameters for NEURON models.
Michael Hines, Sushil Kambampati, Thomas M. Morse.
Yale University School of Medicine,
Neurobiology Department and Computer Science Department,
New Haven, Connecticut.
(www version of a poster presented at the Human Brain Project Annual Conference April 26-27, 2004)
Introduction
The availability of a large number of models in
ModelDB
(Migliore et al 2003),
helps investigators test their
intuition of model behavior and provides building blocks for future modeling
applications to the interpretation of experimental findings. However the NEURON
(Hines and Carnevale 2001) model legacy code entered by publication authors was
generally not developed with presentation as a high priority. The original
code can be difficult to analyze and it sometimes happens that variables are
reset so that the values at run time are different than the first values
indicated in the top of the code. ModelView overcomes these problems by
providing a (run-time state) preview of the properties of a model (anatomy
and biophysical attributes). Having this information available for viewing
in ModelDB lets investigators quickly develop a conceptual picture of the
model structure and compare parameter differences between runs. It makes it
possible to ask detailed questions about the model that would have been
time-consuming to answer without ModelView.
ModelView in action
Example: CA1 pyramidal neuron: as a 2-layer NN and subthreshold synaptic
summation by Poirazi et al 2003
This example demonstrates how ModelView can explore a NEURON model.
We look at the report generated after starting the tool (fig 1).
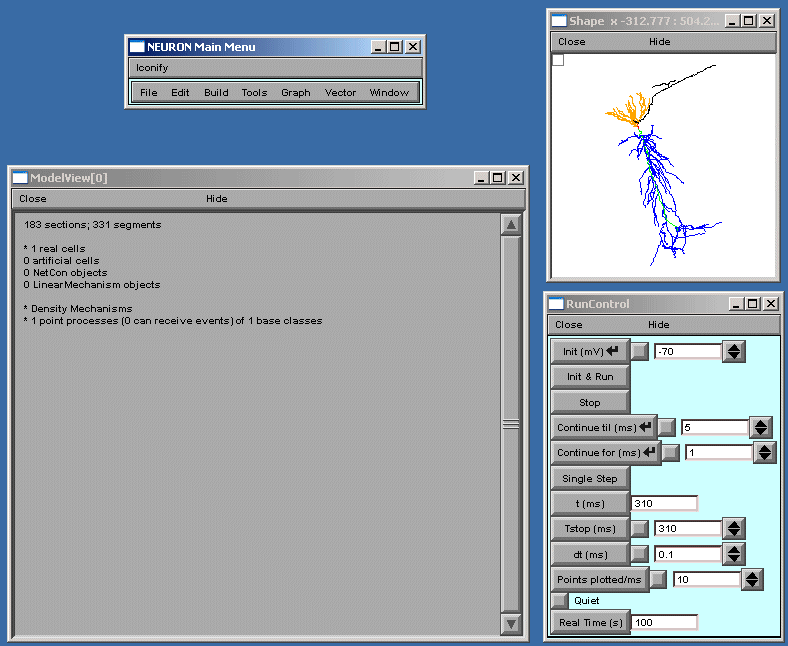
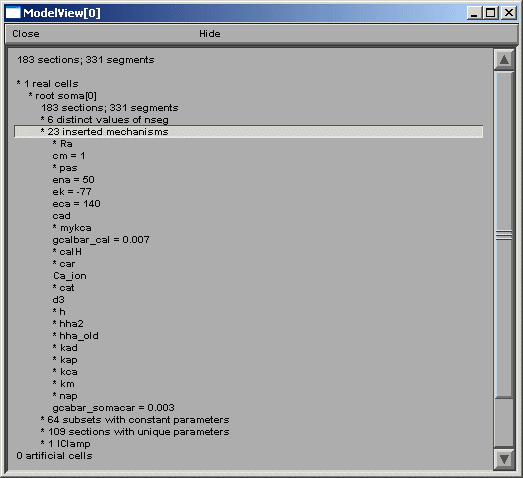
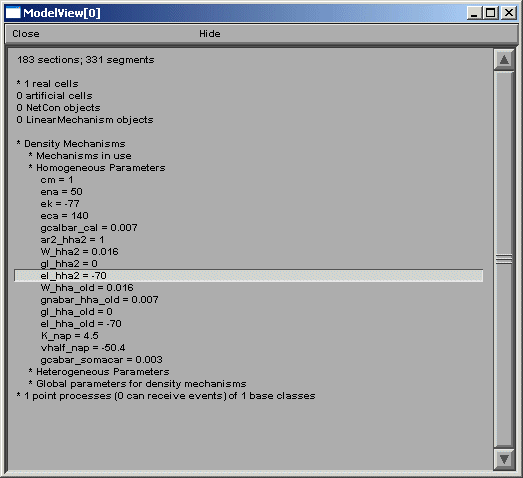
Fig 1
ModelView opens its hierarchical browser with a top-level summary of the model.
In NEURON a cell is divided into sections which are further divided into computational
nodes called segments. The first item in the summary is the total number of sections
and the total number of segments, for all the cells in the model. The second and
final summaries report the numbers of real and artificial cells and density
mechanisms and point processes, the later two include ion-channels, receptors, and
electrodes. The Poirazi example contains 1 real cell and density mechanisms and a
point process. We explore the model by clicking on the asterisk lines to expand the
text tree (or contract if already expanded). Clicking on "* 1 real cells", and then
clicking on the newly appeared "*root soma[0]" displays an analysis of the occurrence
of the duplicate and the unique sets of parameters distributed throughout the cell
(fig 2 left).
Fig 2
After the total number of sections and segments in this particular cell and the related
nseg parameter distribution, the number of inserted mechanisms is reported. Expanding
the number of inserted mechanisms (23 here) lets the investigator know which ion-channels
are used in the model (fig 2 right).
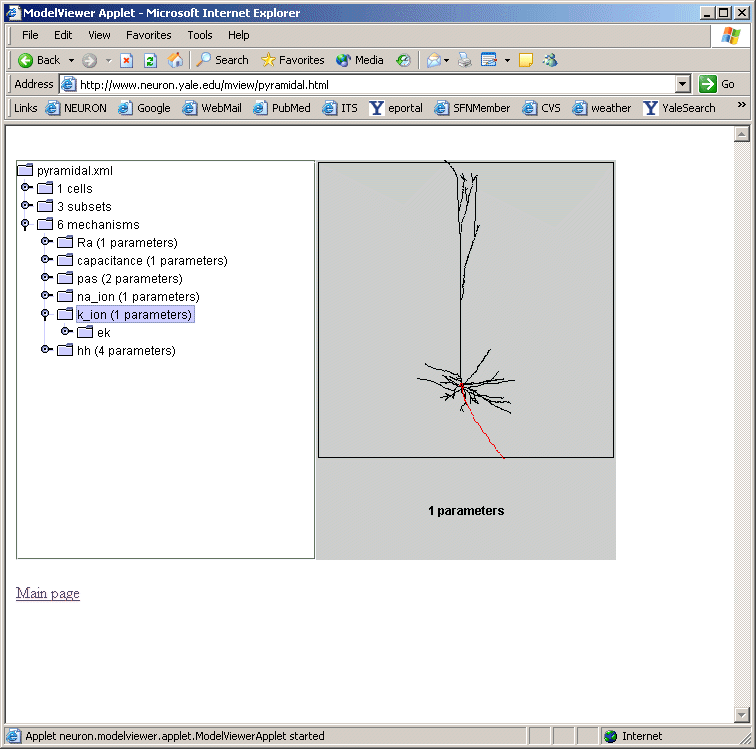
Expanding the "* pas" line (fig 3) shows how ModelView truncates text display when there
are too many values to display with a message "... or more distinct values".
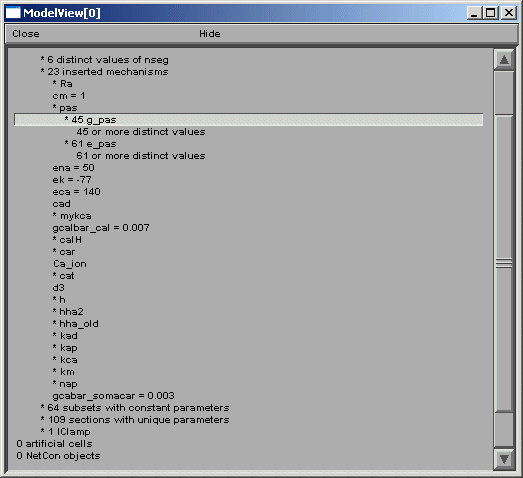
Fig 3
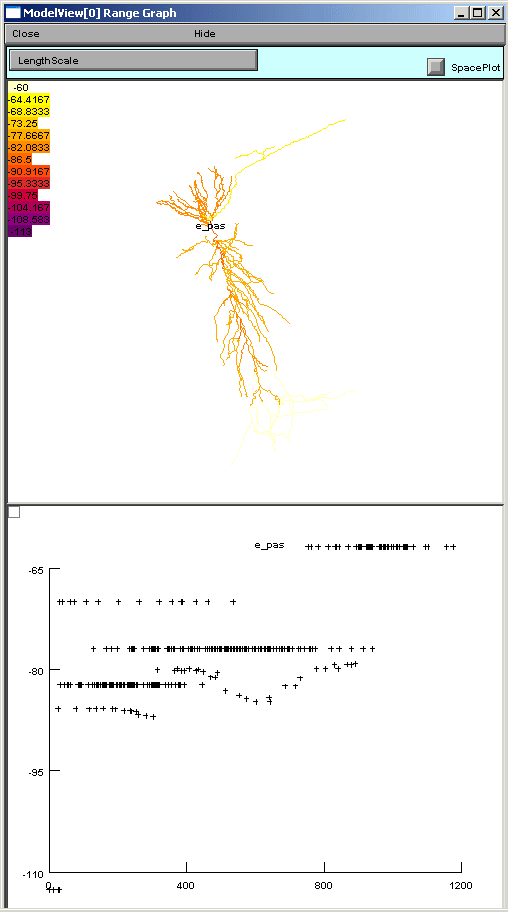
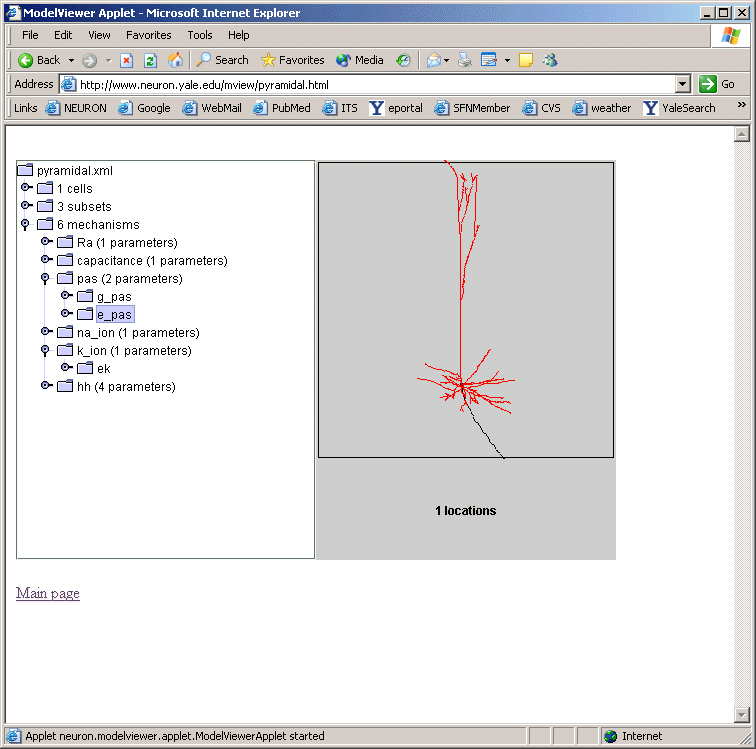
If one of these too-lengthy-to-display items is clicked upon, a graph of the values of
the parameters along an axis indicating distance from the soma and a 3D shape image
with values expressed as color intensities are displayed (fig 4). The image on the
left shows the range graph when g_pas selected (right when e_pas selected).
Fig 4
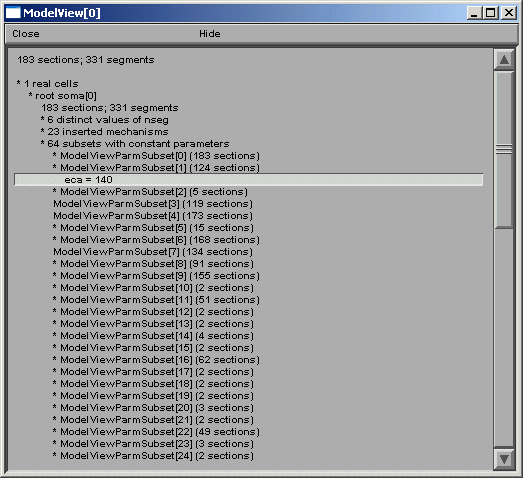
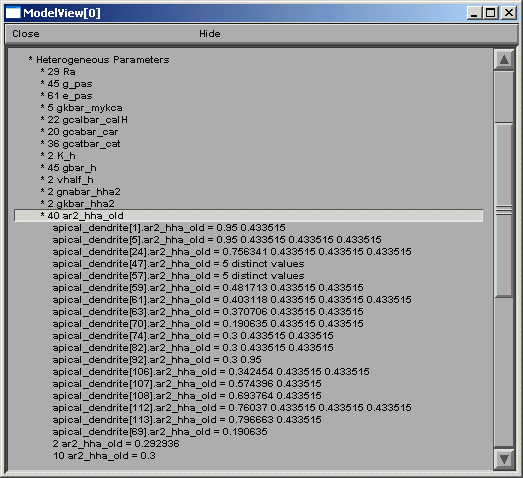
ModelView analyzes the sections to find the sets of sections that share a parameter
(or a set of parameters) set to the same value(s). There are 64 sets of subsets of
constant parameters in this model. The number of sections that have unique values
of a parameter are also calculated and displayed (109 here). These large numbers of
sets are indicative that there are heterogeneous values for some parameters. For a
simpler model, these numbers help identify the combinations of values that are used,
and also their spatial distribution, while for a more complex model, such as this one,
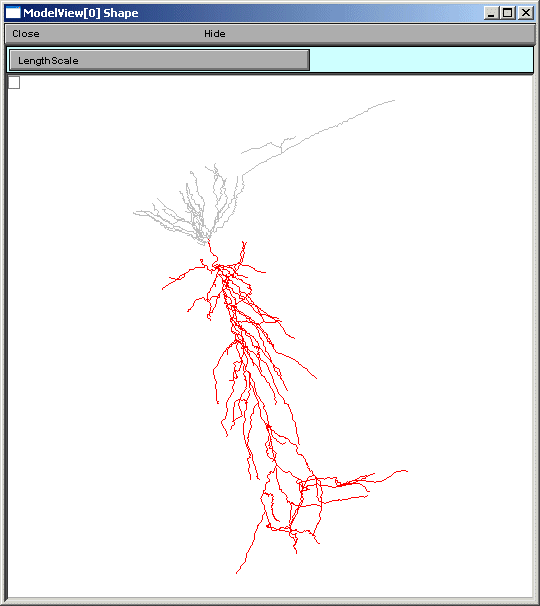
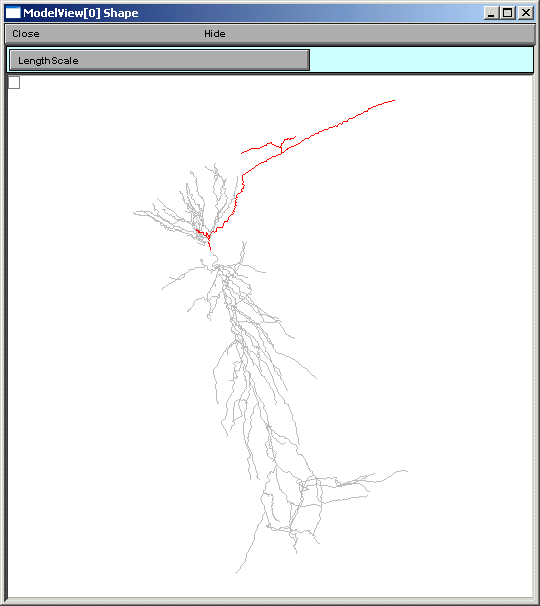
the line items are links to graphs as described above. When a parameter from these
lists is selected (fig 5 left), for example eca = 140, the part of the tree that
contains that value of that parameter is highlighted in a shape plot (fig 5 right).
Fig 5
The final group of top-level items, density mechanisms and point processes will
expand into text trees that summarize and create graphs that display the distribution
of these items. The Homogeneous Parameters item lists the parameters that occur with
just a single value, unlike Heterogenous Parameters, which contain lists of parameters
which take multiple values. When a homogeneous parameter is selected, e.g. el_hha2,
then a graph of the distribution of that parameter is displayed (fig 6).
Fig 6
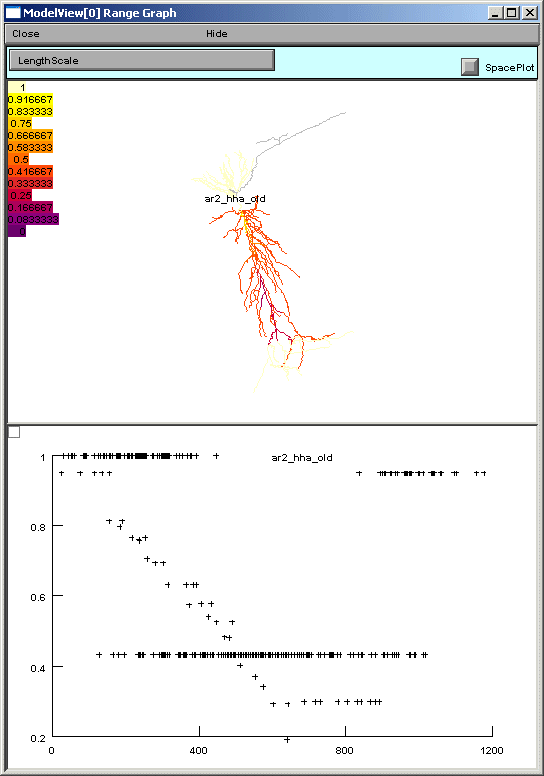
If a heterogeneous parameter is selected, e.g. ar2_hha_old, a term used in a Na channel
activation function, the range plot shows the overall complexity, while the text
provides a place to explore the details of the distribution (fig 7).
Fig 7
Finally, in this example model, there is one point process, a current clamp,
which when selected in the ModelView browser, will graph that it
is positioned at the soma (standard IClamp show shape graph not shown).
ModelView and ModelDB
We plan on incorporating a ModelView window as an alternative window to the model
file browsing window in ModelDB. Investigators will be able to browse the morphology
and biophysical specification for the NEURON models stored in ModelDB. This browser
is under development, but preliminary views (fig 8 below) illustrates its similarity
to the NEURON version.
Fig 8
ModeView and Interoperability Future Goals.
ModelView can output an XML description of the model's morphology and biophysical
specification. ModelView uses MorphML to describe the morphology of the cell.
We are collaborating with the MorphML group (see reference) to evolve the MorphML
specification for interoperability. The biophysical XML specification is under
development and we have plans to provide translation tools to previous and developing
standards. We will continue to refine the XML output to make it suitable for
communication with neuroinformatics tools as well as other modeling software such
as Catacomb and GENESIS. We will adopt, develop through informal collaboration and
communication, and promote NeuroML (see reference) (whose goal is to establish
standard descriptions of computational models that are simulator independent),
BrainML (see reference) (whose goal is to develop standards in the general description
of neuroscience data). We will also attempt to implement the tools required to import
and export models with a third specification, CellML (see reference) (whose purpose
is to store and exchange computer-based biological models.)
Summary
ModelView is a powerful model exploration tool that summarizes and displays information
about NEURON models that can be hard to find if one has only the model code to search
through. The provided summary information and analysis can lead an investigator to ask
questions about the model that would have been time-consuming to answer without ModelView.
In addition XML interoperability tools are under development that will aid investigators
in understanding models and in translating models to simulator platforms different than
the one in which they were originally written.
Acknowledgement
We are grateful for the support of NIH's Human Brain Project Grant number 5P01DC004732-04.
References
BrainML: http://brainml.org/
CellML: http://www.cellml.org/
Hines, M.L. and Carnevale, N.T. NEURON: a tool for neuroscientists. The Neuroscientist 7:123-135, 2001.
Migliore M, Morse TM, Davison, AP, Marenco L, Shepherd GM, Hines ML. ModelDB Making models publicly
accessible to support computational neuroscience. Neuroinformatics 2003 1:131-134
MorphML: http://germain.umemat.maine.edu/faculty/crook/MorphML.html
NeuroML: http://www.neuroml.org/